KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Fall 2024 Vol. 23Next-generation ceramic electrochemical cells for global net-zero goals
Our research team has developed a novel electrode material with maximized catalytic activity, presenting a strategy to enhance power conversion and green hydrogen production efficiency in next-generation ceramic electrochemical cells, thereby advancing the global net-zero society.
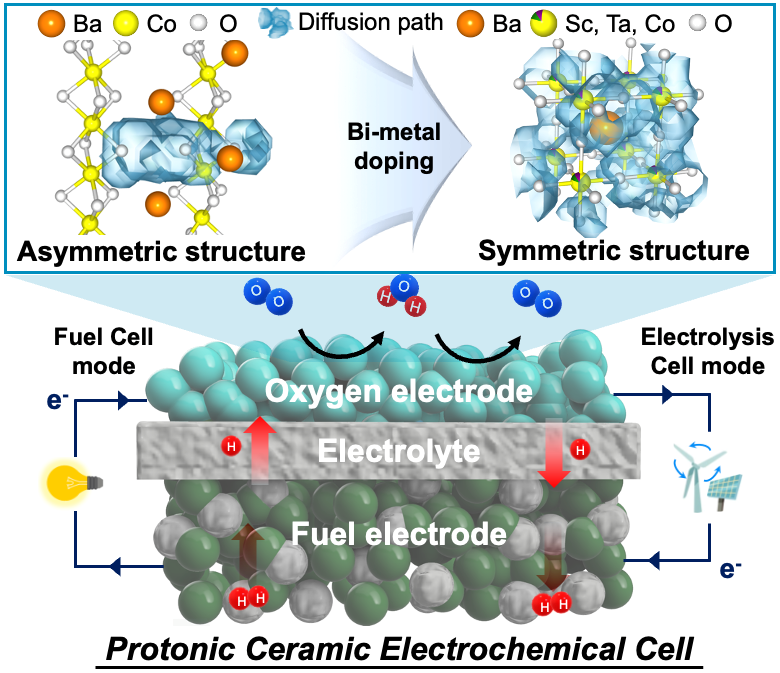
Adhering to the global commitment to 'net-zero' greenhouse gas emissions requires a reduction of carbon emissions through the use and production of hydrogen energy, which has become essential. Among energy conversion technologies usable with hydrogen, protonic ceramic electrochemical cells, capable of high-efficiency power conversion and green hydrogen production, are recognized as a next-generation technology that will drive the future hydrogen energy society.
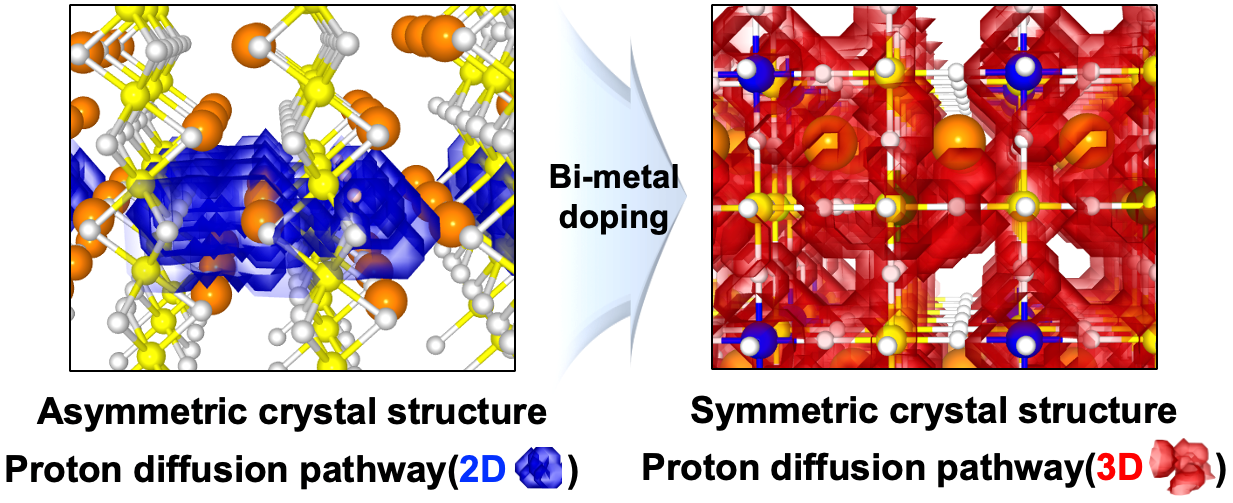
Perovskite oxide-based electrodes with asymmetric structures have faced challenges due to limited proton mobility within the lattice, resulting in low catalytic activity and reduced fuel cell performance. To address this issue, a research team led by Professors Kang Taek Lee and Woochul Jung at KAIST, Dr. Chan-Woo Lee at KIER, and Professor Sun-Ju Song at Chonnam National University selected and doped candidate bi-metal elements, successfully transforming the asymmetric structure, difficult to move, into a symmetric structure and thereby maximizing the proton transport properties (Figure 1). This breakthrough paves the way for the design of high-performance electrodes. Additionally, the team utilized computational chemistry to model and to predict the structure and reactivity of chemical systems theoretically, elucidating the correlation between the crystal structure of the electrode and the proton transport properties.
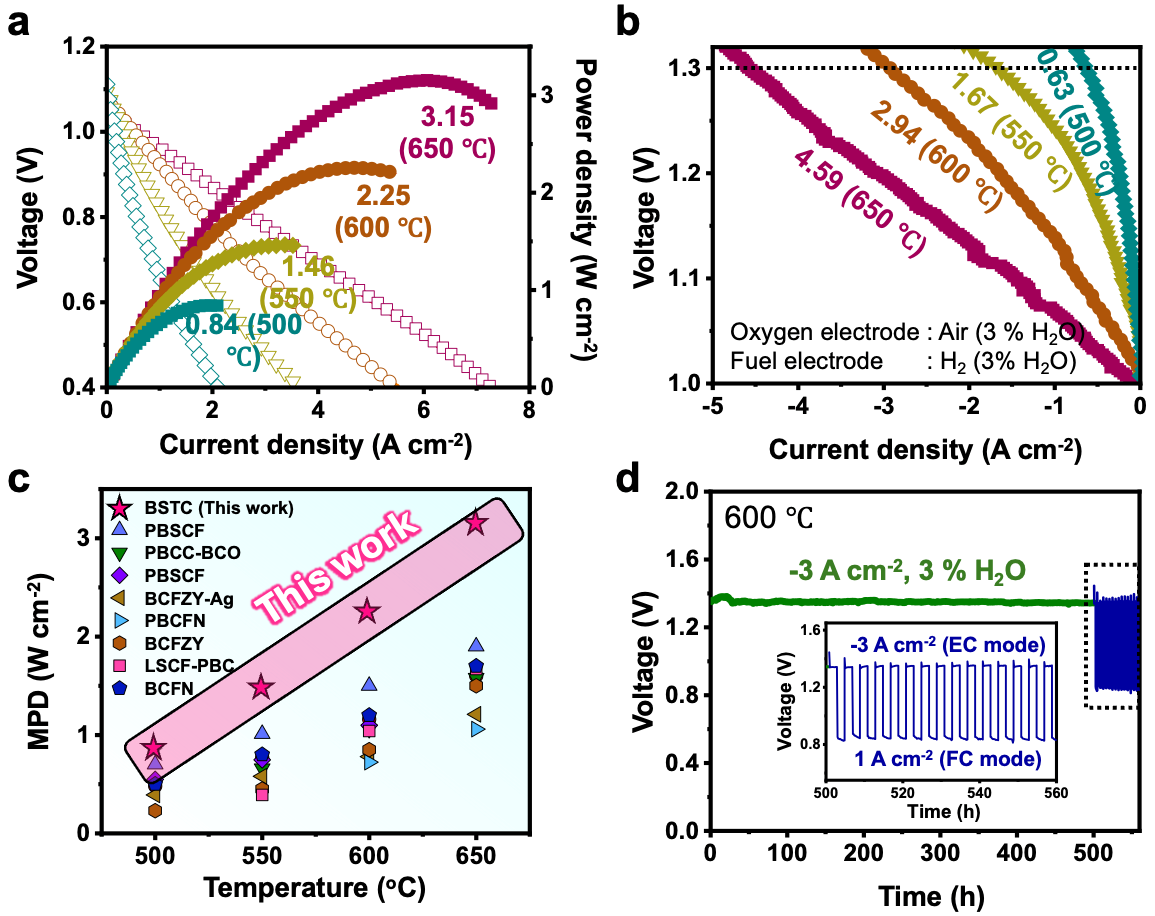
The electrode material developed by the research team has been applied to protonic ceramic electrochemical cells, demonstrating the highest power conversion performance reported to date (3.15 W/cm² at 650°C). Additionally, the material exhibited high production efficiency for green hydrogen, with no carbon dioxide emissions during the production process (approximately 770 ml/cm² per hour at 650°C). After 500 hours of continuous operation, the electrode showed stable performance even during reversible operation (alternating between power and green hydrogen production), proving the effectiveness of the proposed electrode design method (Figure 2).
Professor Kang Taek Lee stated, “The electrode design approach proposed in this study is expected to provide new directions for high-performance power and green hydrogen production in protonic ceramic electrochemical cells. This technology could act as a catalyst for the commercialization of hydrogen production and eco-friendly energy technologies, contributing to the global net-zero goal.
This research was published on April 12, 2024 as a back cover paper in 'Advanced Energy Materials' (IF: 24.4), a leading journal in the energy and materials field. (Title of the paper: On the Role of Bimetal-Doped BaCoO3-δ Perovskites as Highly Active Oxygen Electrodes of Protonic Ceramic Electrochemical Cells).
Most Popular

High-performance and sustainable paper coating material that prevents microplastics
Read more
Next-generation ceramic electrochemical cells for global net-zero goals
Read more
Multi-hand posture rehabilitation for stroke survivors: Rehabilitation system using vision-based intention recognition and a soft robotic glove
Read more
Impacts of new town developments on carbon sinks in the Seoul metropolitan area
Read more
Revolutionizing strength: Hercules artificial muscles 17 times stronger than human muscles
Read more