KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Unveiling the atomic scale sodiation pathway and pulverization-tolerant sodium storage mechanism in copper sulfide
Unveiling the atomic scale sodiation pathway and pulverization-tolerant sodium storage mechanism in copper sulfide
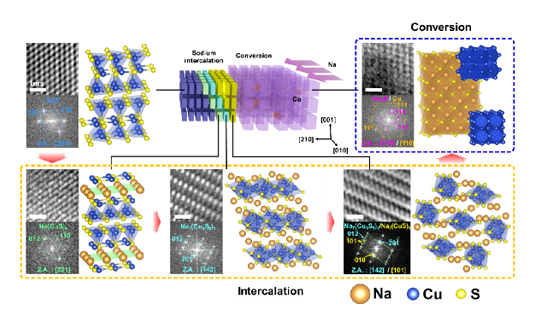
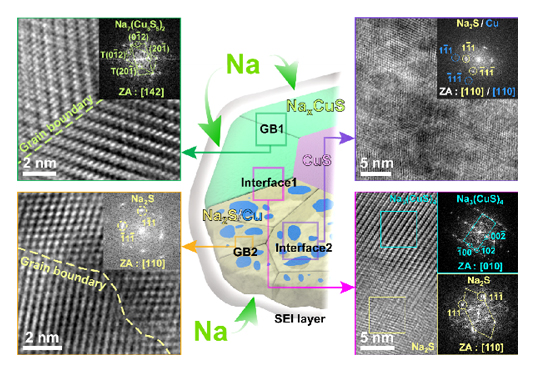
Copper sulfide is suggested as a superior sodium storage material with high capacity and high cyclic stability. To investigate the origin of the high sodium storage performance, in-situ transmission electron microscopy is utilized to visualize the sodium storage process of copper sulfide at the atomic scale and in real time. Copper sulfide undergoes unusual multiple- phase transitions during sodium storage, which enables the formation of semi-coherent grain boundaries and phase interfaces to generate additional active surface area and prevent the pulverization of the particles.
Article | Fall 2019
Lithium ion batteries, which are also referred to as Li-ion batteries or LIBs, are used in various numerous applications including mobile phones. Though they are widely used, large-scale energy storage systems require inexpensive and abundant materials. Hence, sodium ion batteries (SIBs) have attracted enormous attention for their advantages over their lithium counterpart.
However, one main obstacle to the commercialization of SIBs is the lack of suitable anodes that exhibit high capacity and cycling stability of the battery. Intercalation-type materials like graphite, commercialized in LIB, are not acceptable as a sodium storing anode material due to insufficient interlayer spacing leading to low capacity. Thus, high capacity materials with conversion and alloying reactions should be explored. However, in general cases, they undergo a severe capacity degradation accompanying large volume expansions and abrupt crystallographic changes.
A research team of Professor Jong Min Yuk from the Department of Materials Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) discovered that copper sulfide exhibits a stable sodium storage performance with high capacity and cyclic stability despite its conversion reaction.
The research team focused on the fundamental sodium storage mechanism of copper sulfide to unveil the origin of the stable sodium storage. They utilized in-situ transmission electron microscopy to visualize the sodium storage process at the atomic scale and in real time. As a result, it was found that copper sulfide undergoes a gradual crystallographic tuning of CuS-Na(CuS)4-Na7(Cu6S5)2-Na3(CuS)4-Na2S/Cu during sodium storage (Fig. 1). Based on its crystal structure and novel sodium storage mechanism, it forms semi-coherent grain boundaries and phase interfaces to provide additional sodium transport paths and prevent the pulverization of the particles (Fig. 2). This noteworthy finding will potentially contribute to the advancement and commercialization of sodium ion batteries.
The research results were published in Nature Communications (9, 922 (2018) (selected as Editors’ Highlights)) and Advanced Science (6, 1900264 (2019) (selected as the inside back cover).
Most Popular

When and why do graph neural networks become powerful?
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more