KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Novel Si photoelectrodes architecture with nanostructured Au catalysts to reduce CO2 into CO
Novel Si photoelectrodes architecture with nanostructured Au catalysts to reduce CO2 into CO
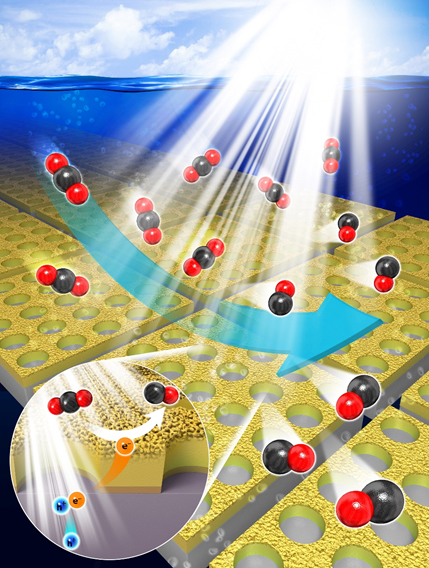
A novel strategy has been proposed for converting carbon dioxide (CO2) into carbon monoxide (CO) by using solar energy and water. Nanostructured Au, with a superior catalytic property for reducing CO2 into CO, is placed on a Si photoelectrode as a co-catalyst. Solar-generated carriers in a Si photoelectrode effectively drive CO2 reduction at the surface of the nanostructured Au, lowering the reaction’s overpotential.
Article | Fall 2017
Carbon dioxide (CO2) has been considered as a main cause of global climate change as its concentration has reached over 400 ppm in the atmosphere. Recently, carbon capture and utilization (CCU) technology has drawn great interest for reducing CO2 concentrations by converting it into value-added chemical products (e.g. carbon monoxide, alcohols, and ethylene) from renewable energy sources (e.g. solar and wind).
Direct CO2 conversion has been considered as a possible technological solution to environmental challenges caused by producing fuels and chemical building blocks (e.g. alcohols and ethylene) from renewable energy sources (e.g. solar and wind).
A photoelectrochemical (PEC) CO2 reduction cell, which is also known as an artificial photosynthesis cell, produces chemical building blocks by using sunlight, water, and CO2 in semiconductor/electrolyte interfaces. However, numerous challenges hinder the development of practical applications of PEC CO2 reduction cells. The most critical obstacles are the high energy required for CO2 reduction due to the stable nature of CO2 molecules. In addition, few semiconductor materials can absorb the visible spectrum of sunlight while also possessing appropriate energetics to reduce CO2.
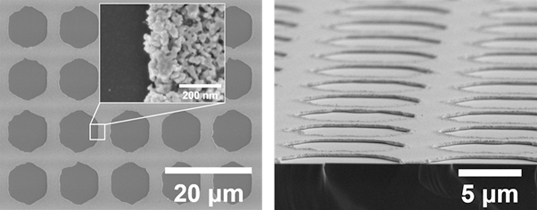
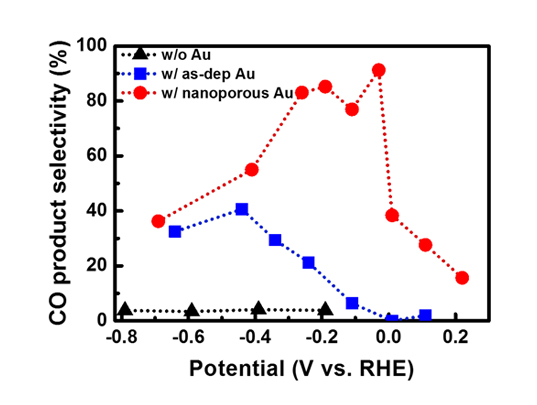
Professor Jihun Oh and his group developed an efficient and selective Si photocathode for CO2 reduction. His group discovered that nanoporous Au thin film formed by electrochemically reducing an anodized Au thin film shows exceptional CO2 reduction activity, along with lowered overpotential and improved selectivity of CO2 reduction. Generally, Au is known as a catalyst for reducing CO2 to CO. CO is a key component of syngas (a mixture of H2 and CO), which is widely used to produce various hydrocarbon products such as diesel and methanol in the chemical industry. The developed nanoporous Au shows over 90% selectivity for producing CO at 480 mV overpotential as compared to bare Au thin film (~70% selectivity for CO at 480 mV overpotential). Their nanoporous Au catalysts uses 200nm-thick thin film, which is 50,000 times thinner than previously-reported 0.1mm-thick Au nanostructure catalyst and is exceptionally cost-effective.
With this discovery, Prof. Oh’s group further proposed a new artificial photosynthetic cell design to use nanoporous Au thin film as a co-catalyst material for solar CO2 reduction. As a solar absorber material, Si semiconductor is used since it is earth-abundant and absorbs most of solar spectrum. The proposed Si photoelectrode with nanoporous Au cocatalysts exhibits an exceptional CO2 reduction property under 1 sun illumination. It achieves 91% CO2 conversion selectivity in order to produce CO at more positive potential (–0.03 V versus reversible hydrogen electrode) than CO2/CO equilibrium potential (–0.11 V versus reversible hydrogen electrode) under 1 sun illumination. Prof. Oh claims that the 90% CO2 conversion rate at more positive potential than equilibrium potential is firstly reported to the best of the team’s knowledge. Prof. Oh believes that their technology provides a general platform to design an efficient photoelectrochemical CO2 conversion cell.
Most Popular

When and why do graph neural networks become powerful?
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more