KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Self-gated cardiac cine MRI towards more comfortable clinical data acquisition
Self-gated cardiac cine MRI towards more comfortable clinical data acquisition
A novel self-gated cardiac cine magnetic resonance (MR) imaging method is proposed. After directly capturing time-resolved cardiac and respiratory motion information from MR signals, high-resolution cardiac cine images can be reconstructed without external motion-monitoring devices.
Article | Fall 2017
Recent studies on MRI applications have reported that clinical assessments using cardiac MRI (CMR) have been steadily increasing. The typical scan time of MRI is relatively long, which makes it vulnerable to object motions and subsequent imaging artifacts. In order to mitigate the motion artifacts, electrocardiogram (ECG) gating and respiratory belts are employed for CMR data acquisition. However, this leads to patient discomfort as additional steps are needed to set up the gating devices. Furthermore, such external gating signals are easily affected by a number of factors, including imaging sequences, blood flow, magnetic field strength, and the condition and position of the attachments.
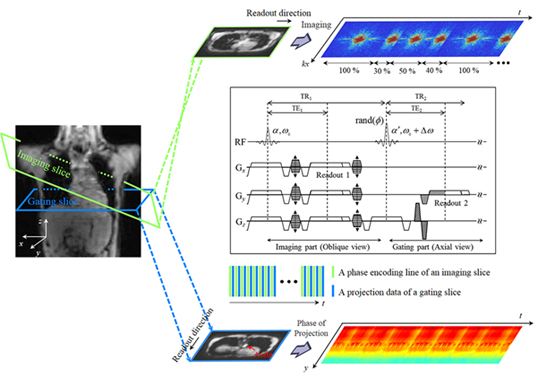
Professor HyunWook Park of the School of Electrical Engineering at KAIST and his team, the Image Computing Systems Lab (ICSL), proposed a novel self-gating method using the MR signal of a separate gating slice for detecting and resolving both cardiac and respiratory motions. In the proposed self-gating method, cardiac motion is estimated from the phase information of the aortic projection data, and respiratory motion is estimated from that of the abdominal projection data.
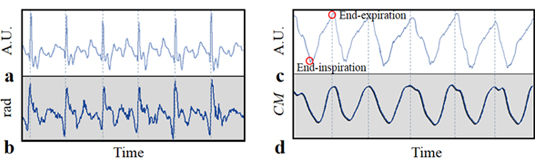
Cardiac motion in the body, which is measured near the diaphragm, causes velocity variation of aortic blood and leads to changes in the MR signal. As the velocities of blood in the descending aorta and cardiac motion are correlated strongly, application of a bi-polar gradient field in the superior-inferior direction for velocity encoding enables extraction of the self-gating signal with respect to cardiac motion. The phase term of the extracted projection data passing through the aorta represents the pulsatile blood flow of the aorta.
Extraction of the self-gating signal for respiratory motion involves the entire region of the projection data instead of specific regions, as was the case for the extraction of cardiac motion. Respiratory motion can be decomposed into the superior-inferior and anterior-posterior directional motions, which are caused by the motions of the diaphragm and abdomen, respectively. Hence, the superior-inferior directional motion from breathing can be estimated by the phase signal amplified by the superior-inferior directional bi-polar gradient field.
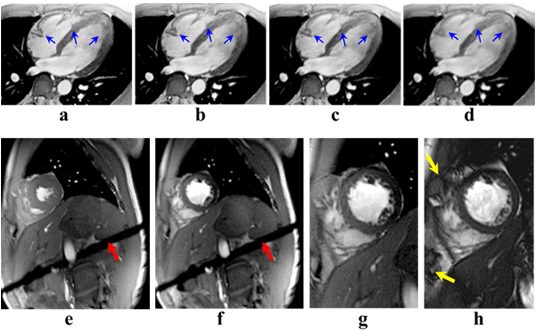
In addition to the new self-gating method, Prof. Park’s team proposed a new MR imaging method, which saves 45% of the imaging time compared to the conventional retrospective gating method. To verify the proposed self-gating method, a simulation and in-vivo imaging at the 3T MRI system were performed. Experimental data and subsequent evaluations from radiologists demonstrated that the proposed method could be a useful self-gating method for multi-phase cardiac imaging, which would offer more comfortable scanning conditions for patients.
The result of the self-gating method was reported in Magnetic Resonance in Medicine (vol 77, issue 3, 2017, DOI: 10.1002/mrm.26204) under the title “Self-gated cardiac cine imaging using phase information.” The same work was awarded the grand prize at the 2016 Samsung HumanTech Paper Award, which was the first grand prize in the award’s 22 years of history.
http://onlinelibrary.wiley.com/journal/10.1002/
https://humantech.samsung.com/
The article of the Samsung HumanTech Paper Award can be accessed here: http://news.joins.com/article/19526826
Most Popular

When and why do graph neural networks become powerful?
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more