KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Development of highly stable zeolite-based CO2 adsorbent under a temperature swing process
Development of highly stable zeolite-based CO2 adsorbent under a temperature swing process
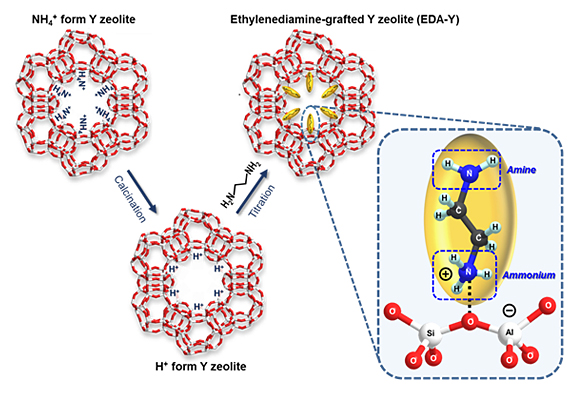
Amine-grafted zeolites are synthesized via a simple acid-base reaction, which adsorbs CO2 effectively and is highly regenerable under a realistic temperature swing CO2 adsorption process.
Article | Fall 2016
Solid adsorbents, including amine-functionalized materials and zeolites, have been extensively investigated for CO2 capture. Amine-functionalized materials have shown promising CO2 uptake in wet flue gas, but suffer from significant amine deactivation due to urea formation under the desorption conditions (e.g., desorption under 100% CO2 at 130oC) of a temperature swing process.
In contrast, zeolites are thermochemically stable but cannot adsorb CO2 from wet flue gas because of the preferential H2O adsorption, which restricts their application in post-combustion CO2 capture.
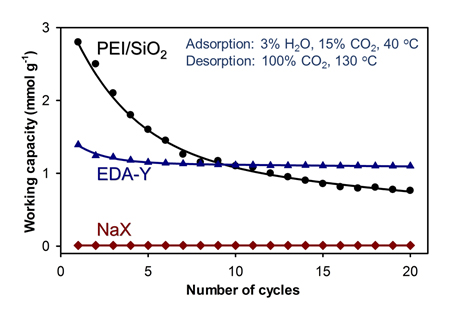
To overcome these limitations, Prof. Minkee Choi’s group in the Department of Chemical and Biomolecular Engineering at KAIST has developed an amine-grafted Y zeolite, which can synergistically combine the strengths of both adsorbent systems. The amine-zeolite hybrid material was synthesized via gas-phase titration of the acid sites in commercial zeolite with ethylenediamine vapor. The amine groups can effectively capture CO2 in wet flue gas, while the strongly co-adsorbed H2O within the hydrophilic zeolite micropores suppresses urea formation (dehydration reaction between amines and CO2) under desorption conditions according to Le Chatelier’s principle. The amine–zeolite hybrid adsorbent retains working capacities higher than 1.1 mmol g1 over 20 TSA cycles.
The new organic-zeolite hybrid adsorbent has a benefit of H2O tolerance during the adsorption of CO2 under wet flue gas compared with purely inorganic zeolites (e.g., molecular sieve 13X). On the other hand, the hybrid material has the benefit of remarkable resistance against urea formation compared with conventional amine-modified materials. Because the adsorbent is synthesized with commercially-available Y zeolite, the material can be economically produced on a large scale. The crystalline zeolite is also more hydrothermally stable than conventional amine supports such as amorphous silica, which can suffer from pore structural collapse under a CO2 stream containing H2O vapor.
An article on this research (entitled “An ethylenediamine-grafted Y zeolite: a highly regenerable carbon dioxide adsorbent via temperature swing adsorption without urea formation”) was published in March 2016 in Energy & Environmental Science.
Reference: C. Kim, H. S. Cho, S. Chang, S. J. Cho, and M. Choi, Energy & Environmental Science, 9, 1803 – 1811 (2016).
Additional link for more information:
http://pubs.rsc.org/en/content/articlelanding/2016/ee/c6ee00601a#!divAbstract (Article)
http://egcl.kaist.ac.kr/
Most Popular

When and why do graph neural networks become powerful?
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more