KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Role of Tau regulating force between microtubules
Microtubules (MTs) are hollow cytoskeletal filaments assembled from αβ-tubulin heterodimers. Tau, an unstructured protein found in neuronal axons, binds to MTs and regulates their dynamics. Aberrant Tau behavior is associated with neurodegenerative dementias, including Alzheimer’s disease. A collaborative research team made up of the labs of Prof. Myung Chul Choi in Bio and Brain Engineering at KAIST and Prof. Cyrus Safinya in Materials and Physics at UCSB reported on a direct force measurement between MTs coated with Tau by using synchrotron small angle X-ray scattering (SAXS). Their study suggests a biological role for regulation by Tau, conferring MT steric stabilization against aggregation either with other biomacromolecules or into tight bundles, preventing loss of function in the crowded axon environment.
Article | Fall 2016
MTs, structural components of the eukaryotic cytoskeleton, are protein nanotubes involved in a range of cell functions, including intracellular trafficking, cell motility, segregating chromosomes, and establishing cell shape.
MTs are composed of straight protofilaments (PFs), which are head-to-tail assemblies of globular αβ-tubulin heterodimers that self-organize, with lateral PF–PF interactions stabilizing the hollow MT. MT structure and assembly dynamics are regulated and functionally differentiated in vivo by the MT-associated-protein (MAP) Tau, which is primarily localized to the axons of mature vertebrate neurons. Although Tau is involved in numerous functions, which remain to be fully elucidated, a well-characterized function of Tau on binding to MTs in mature neurons is in the modulation of MT dynamic instability by enhancing tubulin assembly while suppressing MT depolymerization. This modulation of MT dynamic instability ensures proper trafficking of modifications of Tau, including hyper-phosphorylation and mutations leading to loss of function, which have been implicated in Alzheimer’s disease and a wide range of neurodegenerative disorders, including Fronto-Temporal Dementia with Parkinsonism linked to chromosome 17, Pick’s, supranuclear palsy, and more recently, chronic traumatic encephalopathy in athletes suffering concussions.
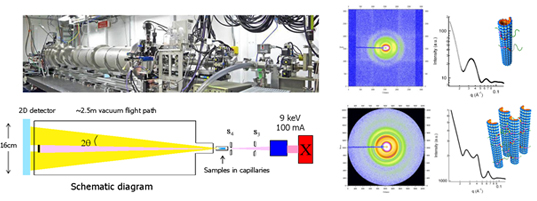
The team has investigated Tau-mediated forces between MTs using synchrotron small angle X-ray scattering (SAXS) subjected to osmotic pressure. This study revealed that Tau fundamentally alters the repulsive forces between MTs in an isoform-dependent manner. In particular, the jump in osmotic pressure required to bundle MTs coated with -M and -L Tau isoforms reveals a gain of function of Tau isoforms with longer N-terminal tails in imparting steric stabilization to individual MTs against bundling.
What is highly significant in this work is the demonstration of the functional property of the charged projection domain (PD) in sterically stabilizing MTs undergoing a transition to a brush state. Previous identification of Tau’s PD was based entirely on its lack of binding affinity to MTs and did not provide insight into its biophysical functions. Steric stabilization against cellular biomacromolecules is essential in preventing MT loss of function in the crowded axon environment.
Furthermore, the conformation of Tau on the MT surface is intrinsically connected to its coverage. The discovery of two distinct conformation states of MT-bound Tau, namely mushroom or brush, further emphasizes the need for future biophysical measurements of MT-bound Tau to be conducted in both regimes. It is interesting to note that the expression of six different Tau isoforms is developmentally regulated, where the fetal brain expresses only the shortest isoform (3RS) but the adult brain expresses around a 1:1 ratio of 3R and 4R Tau. This observation would suggest that the 3RS isoform is likely to be expressed at a higher level in the fetal brain, enabling steric stabilization of fetal MTs at higher coverage, where 3RS Tau would be in a more extended brush conformation. With respect to the adult brain, although the composition of different isoforms is not known at different points along the length of the axon, our finding points to the minimum coverage needed by the longer isoforms (either -M or -L) to impart steric stabilization to MTs.
This minimum coverage required for MT steric stabilization by Tau could also shed light on a possible pathway for neurodegeneration. In the diseased state, Tau is often found to be hyperphosphorylated, with a weaker binding affinity to MTs. MTs lose sufficient Tau coverage to the degree where PDs transition from brush to mushroom and fail to sterically stabilize MTs, and intracellular trafficking on MTs could be significantly hampered by the nonphysiological close-bundled state in the crowded axonal environment.
At high pressures in the MT bundled-phase regime, the polyampholytic nature of Tau resulted in a coverage-dependent electrostatic attraction between MTs. This regime of “tight bundles,” resisted by longer PDs, may conceivably be accessed in vivo in the presence of excess molecular crowding forces. Tight bundles would have negative consequences for organelle trafficking by MTs, where the small MT wall-to-wall spacing, ranging between 3 and 5 nm, would be expected to interfere with motors carrying cargo. In addition to the biological interest in the MT-associated protein Tau as a key component of the axonal cytoskeleton, the directed assembly of MTs by the intrinsically disordered protein Tau is also of broad interest from a biomolecular materials and biophysics perspective. The unique manifestation of short-range attraction and long-range repulsion by Tau of MTs inspires the design of biomaterials with multiple interaction motifs.
Most Popular

When and why do graph neural networks become powerful?
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more