KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Fall 2025 Vol. 25The KAIST team led by Professor Je-Kyun Park has developed a tumor model chip for testing multiple anticancer drugs. This technology could replace animal testing in the future by accurately simulating tissue and organ properties, thus efficiently assessing drug efficacy.
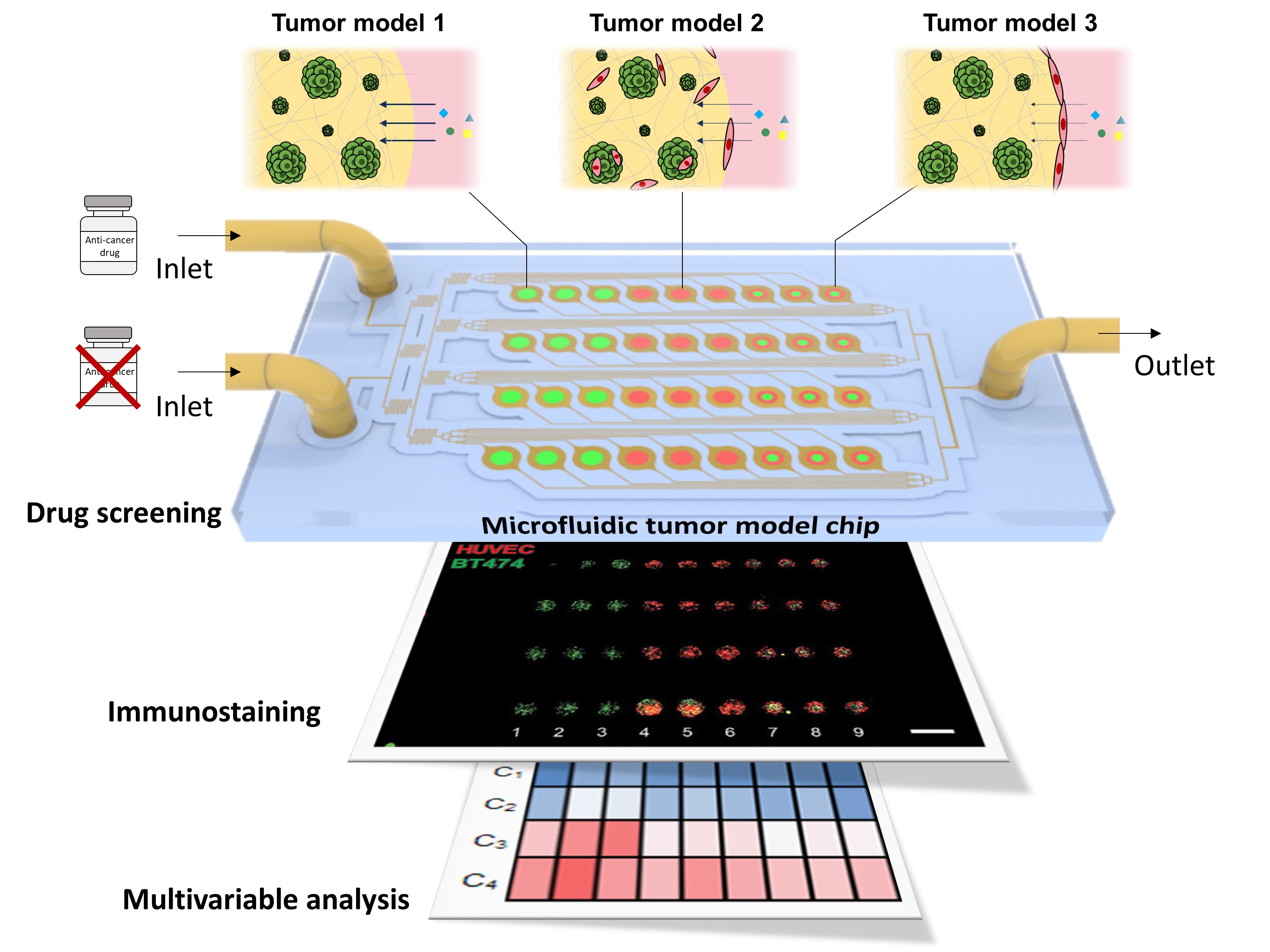
For decades, the pharmaceutical industry has relied on simplified in vitro models—essentially tumor cells grown on flat culture dishes—to evaluate drug efficacy. While convenient, these models fail to replicate the complex drug transport processes in the human body. Drugs administered to real patients must travel through the bloodstream, penetrate vascular barriers, and diffuse through tissues before reaching the tumor. Traditional models overlook these steps, often leading to inaccurate predictions about how drugs will perform in clinical settings.
The research team led by Professor Je-Kyun Park in the Department of Bio and Brain Engineering at KAIST has addressed this critical gap. Using advanced bioprinting techniques that can replicate the complex structures and compositions of tissues and organs in vitro, the team has created 3D tumor models surrounded by vascular barriers—structures that mimic the walls of blood vessels. These models simulate the journey of drug molecules as they diffuse through the vascular walls and infiltrate the tumor mass.
Furthermore, the bioprinted models are integrated into a lab-on-a-chip platform, a microfluidic device that allows researchers to precisely control the flow of fluids. This setup recreates the dynamic culture environment of cells, enabling more accurate and reliable drug testing at different drug concentrations.
In a remarkable demonstration, the researchers printed 36 tumor models with three different compositions on a single chip. The multiple tumor models were cultured under medium flow to form the vascular barriers surrounding tumor masses, which are key structures for mass transport. They then introduced four concentrations of anticancer drugs, running 12 simultaneous experiments. This high-throughput system not only speeds up testing but also enables detailed multivariable analysis, providing insights that would be unattainable using conventional methods.
One of the study’s key findings was the significant impact of vascular barriers on drug efficacy. The researchers observed how these barriers hindered drug transport into the tumor, resulting in significant differences from conventional models that ignored these critical structures. This finding underscores the limitations of existing in vitro systems and highlights the potential of KAIST’s platform to bridge the gap between laboratory research and clinical reality. By integrating bioprinting and lab-on-a-chip technologies, the researchers were able to develop an advanced in vitro platform that takes into account variables such as model complexity, replicates, and throughput, enabling more reliable drug evaluations.
“This achievement represents a major advance in drug evaluation based on the microfluidic cell culture and analysis platform,” said Professor Park. “By recreating the complex environment of human tissues, we can make more accurate predictions of how drugs will work in real patients. Our platform also has the potential to reduce reliance on animal testing and provide a more ethical and sustainable alternative.”
This study, with Dr. Gihyun Lee as the first author, was recently published in Advanced Healthcare Materials under the title “Bioprinted multi-composition array mimicking tumor microenvironment to evaluate drug efficacy with multivariable analysis” (https://doi.org/10.1002/adhm.202303716). In addition, the paper was simultaneously selected by Wiley-VCH publishers for the special sessions “Hot Topic: Tumors and Cancer” and “Hot Topic: Microfluidics”. The research was supported by the National Research Foundation of Korea.
Most Popular

A New solution enabling soft growing robots to perform a variety of tasks in confined spaces
Read more
Towards a more reliable evaluation system than humans - BiGGen-Bench
Read more
Development of a compact high-resolution spectrometer using a double-layer disordered metasurface
Read more
AI-Designed carbon nanolattice: Feather-light, steel-strong
Read more
Dual‑Mode neuransistor for on‑chip liquid‑state computing
Read more