KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Structural contribution of oxide electrocatalysts to efficient oxygen evolution for water splitting
Structural contribution of oxide electrocatalysts to efficient oxygen evolution for water splitting
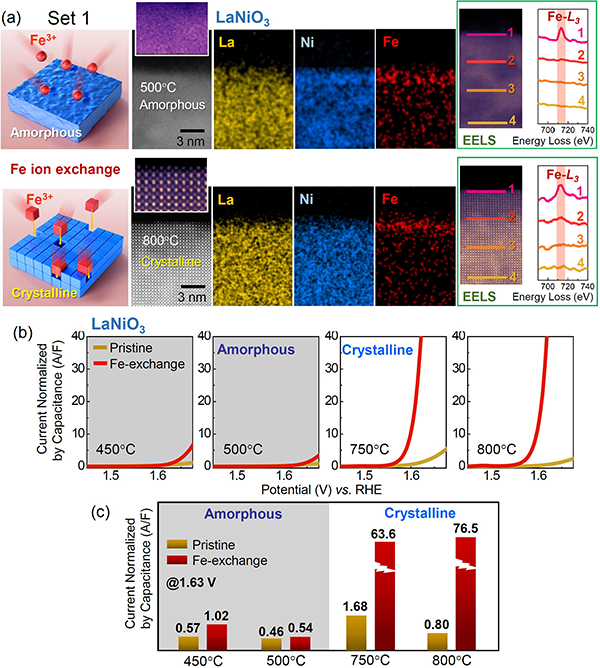
By adopting two distinct Fe-doping methods in amorphous and crystalline nickel oxides, this work finds that structural variations even on the angstrom scale in crystalline catalysts with identical compositions can drastically affect the final oxygen-evolution performance.
Article | Fall 2022
A change in the chemical composition of an electrocatalyst always accompanies variations of the electronic state, which is a key parameter affecting the charge transfer and thereby the overall oxygen evolution reaction (OER) catalytic performance. While this compositional dependency of the electronic states is well recognized, it appears that the structural dependency typically has been overlooked. In particular, as the synthesis of many nanoscale catalysts is frequently carried out at low temperature, the local structure at catalytically active sites can vary considerably in a thermodynamically non-equilibrium state, strongly depending on the thermal history during synthesis. The major contribution made by doping to improve the catalysis step is thus fairly difficult to pinpoint unless the structure adjacent to the dopants is precisely determined. In this regard, this type of structural ambiguity is a serious obstacle to those seeking to clarify the role of doping in many oxides and hydroxides.
To compare the effects of the crystal structure on the overall OER activity systematically and to decipher the structurecomposition correlation during OER catalysis when Fe is added to nickel-based oxides, Prof. Sung-Yoon Chung’s group in the Department of Materials Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) has utilized lanthanide nickelate films having two distinct degrees of crystallinity: perovskite-type crystals with the (001) surface vs. amorphous counterpart phases. Furthermore, two different Fe-doping processes are adopted: solid solutions containing Fe through annealing at a high temperature and an electrochemical Fe exchange at room temperature. Three sets of experimental results along with atomic-scale direct analyses show that no OER catalysis boost on a single-order scale is observed in amorphous nickelates regardless of the Fe-doping method (Figure 1). In stark contrast, exceptionally high OER activity is identified in room-temperature Fe-exchanged crystalline films, while such a remarkable improvement in the OER catalysis step is not achieved in Fe solid-solution crystalline films. These findings along with molecular dynamic simulations and density-functional-theory calculations suggest that symmetry-breaking lattice distortion at the Fe sites with preservation of the crystalline framework makes a critical contribution to attaining higher Fe 3d states near the Fermi level for easier charge transfer, representing a notable enhancement of OER catalysis in perovskite nickelates. Our study highlights the fact that the atomic structure at catalytically active sites provides crucial information leading to a precise understanding of the higher OER efficiency in crystalline oxides.
This work was published in Energy & Environmental Science (vol. 15, 610620 (2022)) and was featured on the inside cover of the February issue.
Additional links for more information:
https://pubs.rsc.org/en/content/articlelanding/2022/ee/d1ee01826d
Most Popular

When and why do graph neural networks become powerful?
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more