KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Grain boundaries as exceptionally active sites for oxygen electrocatalysis in perovskite oxides
Grain boundaries as exceptionally active sites for oxygen electrocatalysis in perovskite oxides
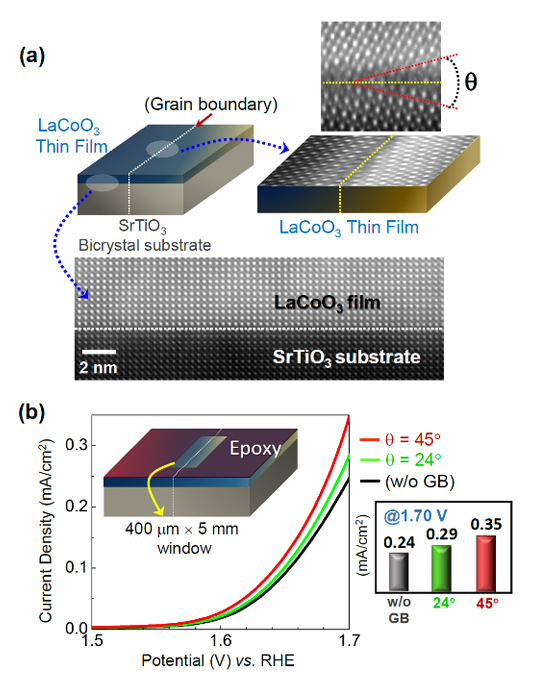
By using both sintered polycrystals with various grain sizes and thin films on bicrystal substrates, it is directly demonstrated that surface-terminating grain boundaries in LaCoO3 and LaMnO3 are exceptional in oxygen evolution electrocatalysis, showing more than an order of magnitude higher activity.
Article | Spring 2019
The overall reaction involved with multiple charge transfers and atomic adsorption/ desorption during oxygen evolution and reduction has a comparatively large activation barrier. Therefore, utilization of low-cost and highly-efficient electrocatalysts to reduce the barrier has been an important issue for rapid redox reactions in fuel cells and metaloxygen batteries as well as water-splitting devices.
Including the initial notable works in the 1980s, many experimental and theoretical studies on the ABO3-type perovskite catalysts have dealt with the number of d-electrons of B-site cations, the bond strength of BOH, the position of the O 2p band center, the effect of oxygen vacancies, and the role of lattice oxygen redox in efforts to account for the crucial contributions that correlate with the high catalytic activity during the oxygen evolution reaction (OER). Although various structural and electronic factors should be considered to precisely depict the catalytic behavior of perovskites, it appears to be accepted in general that relative difference of energy level between the 3d orbitals of B-site cations and the 2p orbitals of oxygen anions play an important role in significant promotion of charge transfer between [BO6] at the surface and adsorbates, resulting in much higher OER efficiency at a lower overpotential.
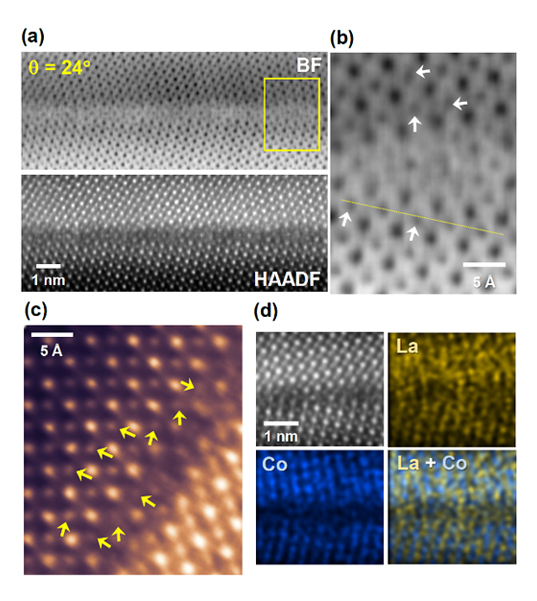
In stark contrast to the [BO6] octahedra in the bulk, most [BO6] units at grain boundaries (GBs) in polycrystals have severely distorted geometric configurations of B and O. As a result, it can be reasonably anticipated that the electronic states of transition-metal 3d and oxygen 2p orbitals at GBs considerably differ from those in the bulk. In this regard, a combination of selective analysis of the OER characteristics at GBs and observation of the grain-boundary atomic structure can provide direct and insightful information that has not been offered in previous studies to elucidate the correlation between the physical structure, the electronic states of orbitals, and the resultant OER catalysis properties.
Prof. Sung-Yoon Chung’s group in the Department of Materials Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) has successfully demonstrated that surface-terminating grain boundaries are exceptionally active sites for the OER. In particular, when they compared the OER activity between the (001) surface and the 24o-tilt grain boundary on a LaCoO3 bicrystal film, they observed three orders of magnitude higher OER current density at GBs. With the aid of atomically resolved scanning transmission electron microscopy, they successfully visualize both significant Co displacement and strong distortion of oxygen in [CoO6] octahedra near grain boundaries. A series of ab initio density functional theory calculations, considering all the possible atom displacements, also reasonably suggest a substantial increase of the density of electronic states (DOS) near the Fermi level, indicating much easier charge transfer during the OER.
The experimental and theoretical results from the present work offer very significant implications in addition to clarifying the impact of GBs on the remarkably efficient OER performance in perovskite oxides. First, this study has critically and unequivocally demonstrated that GBs are exceptionally active for the OER in in LaCoO3 and LaMnO3. As in the Jahn-Teller theorem, such symmetry-broken atomic displacements in [CoO6] octahedra can induce substantial nondegeneracy of the Co 3d-orbital levels including the eg level. In addition, these results can support the significant OER catalytic performance observed in many amorphous oxide materials. Disordered oxygen polyhedra in amorphous oxides may be equivalent to the extreme case of very strong distortion of octahedra. Consequently, if the number of transition metals, which are active site for the OER catalysis, does not vary on the surface of amorphous particles, the structural disordering can promote the nondegeneracy of metal 3d-orbital levels and thereby enhance the lower p-to-d charge transfer.
This work was published in Advanced Energy Materials (2018, 8, 1802481), and also highlighted on the Front Cover of the journal.
Additional links for more information:
https://onlinelibrary.wiley.com/toc/16146840/2018/8/34
https://sites.google.com/site/atomicscaledefects/home
Most Popular

When and why do graph neural networks become powerful?
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Development of a nanoparticle supercrystal fabrication method using linker-mediated covalent bonding reactions
Read more