KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Promotion of gas hydrate nucleation in clay-rich sediments
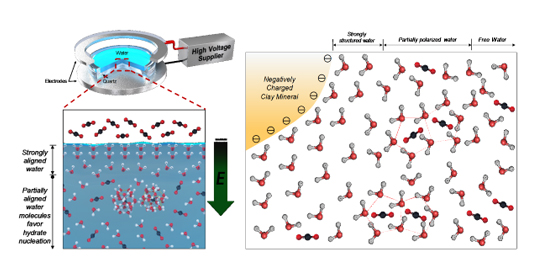
Clay minerals have negatively charged surfaces which polarize the absorbed water molecules on their clay surfaces and such alignment of water molecules promotes the nucleation of gas hydrate.
Article | Spring 2019
Natural gas hydrates, which are frequently found in oceanic clay-rich sediments, are considered prospective energy resources owing to their abundance, and also as potential triggers and/or accelerators for geological hazards and climate change. Due to the isomorphous substitution of cations in clay mineralogy, the surfaces of natural clay minerals are negatively charged and thus unavoidably generate physicochemical interactions with water.
Small pore size and adsorbed cations of the clays are known to limit gas hydrate formation due to the reduced activity of water in such environment. However, it is still unclear why and how natural gas hydrates are widely found in clay-rich sediments in spite of factors that limit gas hydrate formation such as small pore sizes and high salinities of clay-rich sediments.
A research team (GeoEnergy Laboratory) at the KAIST Department of Civil and Environmental Engineering, led by Prof. Tae-Hyuk Kwon, has reported for the first time the role of polarized water molecules adsorbed on clay surfaces in gas hydrate formation. It is challenging to identify the sole contribution of the polarized water molecules on negatively-charged clay surfaces because the test results always involve the effects by other factors, such as by cations and small pore size. A key to their breakthrough was to apply an external electric field to water, which polarizes the water molecules as they are on the negatively-charged clay surfaces. Inspired by previous studies of electrofreezing studies to solely investigate the role of external electric field on ice nucleation, the research team designed and conducted gas hydrate nucleation tests while applying an external electric field to the deionized water.
The research team found that the presence of polarized water molecules on clay surfaces clearly promoted gas hydrate nucleation. When water molecules were polarized parallel to the external electric field, it was found that gas hydrate nucleation occurred significantly faster, due in part to the partial breakage of the hydrogen bonded water clusters and the lowered thermal energy of water molecules (Figure 1A). Further, inhibition of gas hydrate formation was observed when an electric field was applied prior to gas dissolution; this was likely due to the hindrance in gas uptake because of the presence of strongly aligned water layers at the water-gas interface (Figure 1B).
Their findings expand the understanding of the formation habits of naturally occurring gas hydrates in clay-rich sedimentary deposits, and provide insights into gas production from natural hydrate deposits. This research was published on February 3rd, 2018 in Environmental Science and Technology with the title of “Effect of electric field on gas hydrate nucleation kinetics: Evidence for the enhanced kinetics of hydrate nucleation by negatively charged clay surfaces.” (DOI: 10.1021/acs.est.7b05477).
Most Popular

When and why do graph neural networks become powerful?
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Development of a nanoparticle supercrystal fabrication method using linker-mediated covalent bonding reactions
Read more