KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Bacteria engineered to produce and secrete free heme
Secretory production of free heme was achieved using an engineered E. coli strain. This strategy will expedite the efficient production of free heme to serve as a bioavailable iron-supplying agent and an important prosthetic group of multiple hemoproteins for medical uses.
Article | Spring 2019
Researchers of KAIST have defined a novel strategy for the secretory production of free heme using engineered Escherichia coli (E. coli) strains. They utilized the C5 pathway, the optimized downstream pathways, and the heme exporter to construct the recombinant microorganism producing extracellular heme in the fed-batch fermentation. This is the first report to extracellularly produce heme using engineered E. coli. This study, led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST, was published in Nature Catalysis.
Heme, an organometallic compound complexed with a ferrous ion, is an essential molecule delivering oxygen in the blood of many animals. It is also a key component of electron transport chains responsible for the cellular respiration of aerobic organisms including diverse bacteria. Heme is now being widely used as a bioavailable iron-supplying agent in the healthcare and dietary supplement industries. The demand on heme and the need for efficient production of this compound are continuously growing.
Previously, many researchers attempted to produce free heme using engineered E. coli. However, none of the studies were successful in producing free heme extracellularly, requiring an additional step to extract the accumulated heme from cells for subsequent uses. The secretion of heme in the form of heme peptides or proteins also requires an extraction step to isolate the free heme from the secreted products. Thus, the secretory production of free heme is an important task for economical production that is suitable for use in humans.
Although some researchers managed to produce intracellular heme using the recombinant E. coli strains, its final titer was extremely low, due to the usage of sub-optimal metabolic pathways. Furthermore, the addition of precursors, l-glycine and succinate, was deemed undesirable for massive industrial production. Thus, it is necessary to construct an optimized heme biosynthetic pathway to enable the efficient production of heme and examine the consequent secretion of free heme.
To address this issue, Professor Lee used multiple strategies to produce extracellular free heme by enhancing its biosynthesis in E. coli. First, the capacities of the C4 and C5 pathways to produce aminolevulinate (ALA) without feeding precursors were examined. After confirming the superior performance of the C5 pathway over the C4 pathway, the metabolic genes of the C5 pathway and downstream pathways for heme biosynthesis were overexpressed. Then, metabolic pathways were optimized by adjusting the expression levels of the relevant genes and disrupting the putative heme degradation enzyme encoded by the yfeX gene.
Consequently, the resulting engineered strain secreted a significant amount of heme to the medium. Subsequent optimization of the cultivation conditions and the supplementation of nitrogen sources further increased both titer of the total free heme and the fraction of free heme secreted to the medium. Finally, overexpression of the ccmABC genes encoding the heme exporter further enhanced the production and secretion of heme, producing the highest titer of heme both intracellularly and extracellularly from glucose.
Professor Lee said, “The eco-friendly and sustainable chemical industry is the key global agenda every nation faces. We are conducting research to bio-synthesize high concentrations, high yields, and high productivity in natural products. This novel technology will serve as an opportunity to advance the biochemical industry moving forward.”
Most Popular

When and why do graph neural networks become powerful?
Read more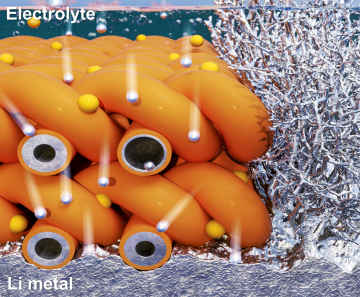
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more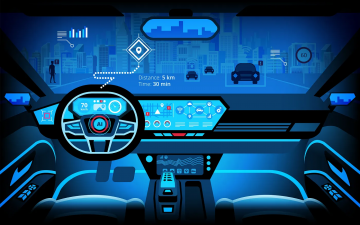
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Development of a nanoparticle supercrystal fabrication method using linker-mediated covalent bonding reactions
Read more